Background: Recurrence of disease is seen in some patients post CD19 targeted CAR-T cell therapy. There is a need to develop CAR-specific efficacy risk-classification systems as established parameters of disease relapse are not predictive. In the Phase-2 IMAGINE study, multiple variables that may predict clinical outcomes were studied and the relevant clinical, laboratory and translational information were integrated.
This abstract reviews the development of the Bengaluru Score as a predictive prognostic score for clinical efficacy outcomes based on discovery cohort of patients treated with varnimcabtagene autoleucel (IMN-003A).
Methods: Multiple characteristics for disease progression were reviewed (n=36) and risk stratified as: 1) Baseline parameters (age, lines of treatment, bridging therapy, cytogenetics, blasts, total metabolic tumour volume (TMTV), sum of perpendicular diameter (SPD), refractory, relapse within 6m, laboratory parameters - haemoglobin, neutrophil count, lymphocyte count, platelet count, CRP, LDH, ferritin, baseline interleukin-6 (IL-6), baseline total T cells, CD4, and CD8 subsets in peripheral blood); 2) Apheresis (total T cells, naïve / central memory in CD4 and CD8 subsets); 3) Final Product characteristics [total CAR+ cells, naïve / central memory in CD4 and CD8 subsets, CD8 (naïve & central memory subsets / tumour burden (TMTV and SPD), product doubling time] and 4) Post Infusion (CAR peak & persistence, B cell aplasia, hypogammaglobulinemia, infections and intravenous immunoglobulin (IVIg) replacement).
These parameters were analyzed using chi-square and appropriate multivariant odds ratio (OR) to examine the association with disease progression. Independent risk factors were identified and weighted score allocated for OR ≥10 to obtain a multifactorial prediction model - The Bengaluru Score. The specificity and sensitivity of this model was then studied in the discovery cohort.
Results: In the IMAGINE study, with ORR 80.9% at primary endpoint (n=17/21) and median PFS not reached (range 12 to NR), relapse was seen in 37.5% (n=9/24) pts with median PFS of 178 days (range 28 to 319).
Multivariant analysis of these parameters showed some statistical significance for following variables: Baseline Platelet count <200 x 10 9/L (overall); Final Product doubling time ≥1 day (B-ALL); Baseline SPD ≥2000 mm 2, Hb <12 g/dL, Platelet count <200 x 10 9/L and post infusion IVIg replacement (B-NHL), Of these variables, statistically significant association was seen with Final Product doubling time ≥1 day (B-ALL) and Baseline SPD ≥2000 mm 2 with Hb <12 g/dL and Platelet count <200 x 10 9/L (B-NHL). The impact of IVIg replacement on efficacy needs further study.
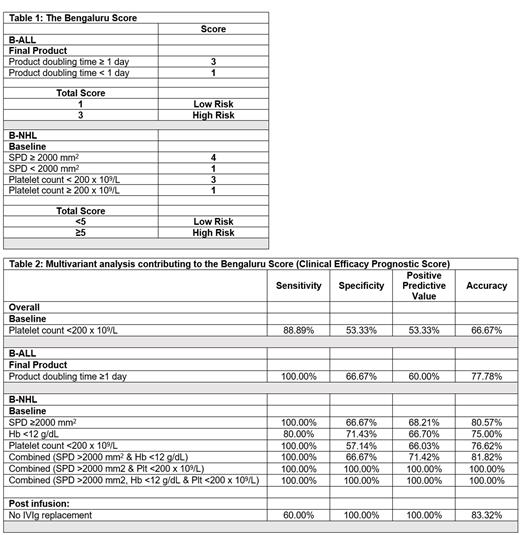
A multiple factor-based prognostic score system - The Bengaluru Score was developed classifying patients into B-ALL low-risk (1 point) and high-risk (3 points) and for B-NHL low risk (<5 points) and high-risk (≥5 points), Table 1.
The Bengaluru Score when applied to discovery cohort in the IMAGINE study was able to risk stratify patients for disease progression with Sensitivity, Specificity, Positive Predictive Value and Accuracy of 100.00%, 66.67%, 60.00% and 77.78% respectively (for B-ALL) and 100.00%, 100.00%, 100.00% and 100.00% respectively (for B-NHL), Table 2.
Conclusions: The Bengaluru Score is probably the first predictive prognostic score for clinical efficacy outcomes and is based on multivariant statistical analysis in the discovery cohort of patients in the IMAGINE study integrating clinical, laboratory and translational data with a very high sensitivity, specificity and accuracy. The score highlights the role of overall T cell expansion in the final product for B-ALL (but not the T N+CM subsets on its own) and for B-NHL, disease burden and haematological reserve. This suggests that earlier use of varnimcabtagene autoleucel may positively influence efficacy outcome but needs to be proven in the real-world setting. The Bengaluru Score can guide informed treatment decisions and evolve clinical strategies, such as reinfusion, CAR-T targeting a different antigen or allogeneic transplant (if applicable), to reduce the risk of relapse / disease progression in high-risk patients. The Bengaluru Score needs validation in a larger cohort of patients following treatment with varnimcabtagene autoleucel and other CD19 targeted CAR-T cell therapies.
Disclosures
Kamat:Immuneel Therapeutics Private Limited: Current Employment. Arasu:Immuneel Therapeutics Private Limited: Current Employment. Akheel:Immuneel Therapeutics Private Limited: Current Employment. Anand:Immuneel Therapeutics Private Limited: Current Employment, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal